

The electronegativity difference between beryllium and chlorine isn't enough to allow the formation of ions.īeryllium has 2 outer electrons because it is in group 2. The only simple case of this is beryllium chloride, BeCl 2. Two electron pairs around the central atom How this is done will become clear in the examples which follow.ĭon't panic! This is all much easier to do in practice than it is to describe in a long list like this one! You know how many bonding pairs there are because you know how many other atoms are joined to the central atom (assuming that only single bonds are formed).įor example, if you have 4 pairs of electrons but only 3 bonds, there must be 1 lone pair as well as the 3 bonding pairs.įinally, you have to use this information to work out the shape:Īrrange these electron pairs in space to minimise repulsions. Work out how many of these are bonding pairs, and how many are lone pairs. Now work out how many bonding pairs and lone pairs of electrons there are:ĭivide by 2 to find the total number of electron pairs around the central atom. For example, if the ion has a 1- charge, add one more electron. (This allows for the electrons coming from the other atoms.)Īllow for any ion charge. That will be the same as the Periodic Table group number, except in the case of the noble gases which form compounds, when it will be 8.Īdd one electron for each bond being formed. Write down the number of electrons in the outer level of the central atom.
HYDRONIUM ION BONDING AND HYBRIDIZATION HOW TO
If you are working to a UK-based syllabus for 16 - 18 year olds, and haven't got copies of your syllabus and past papers follow this link to find out how to get them.įirst you need to work out how many electrons there are around the central atom: It is important to know exactly which molecules and ions your syllabus expects you to be able to work out the shapes for in this part of the syllabus. At that point, learn the ones your syllabus wants you to know.

When you deal with transition metal chemistry, you will be expected to know the shapes of some ions formed by transition metals, but not to work them out. The method will, however, cope with all the substances that you are likely to meet in this section of the syllabus. Warning: This method won't work without some modification for many ions containing metals, and no simple method gives reliable results where the central atom is a transition metal.

But this is all very tedious! You can get exactly the same information in a much quicker and easier way for the examples you will meet if you are doing one of the UK-based exams for 16 - 18 year olds. You can do this by drawing dots-and-crosses pictures, or by working out the structures of the atoms using electrons-in-boxes and worrying about promotion, hybridisation and so on. How to work out the number of electron pairs
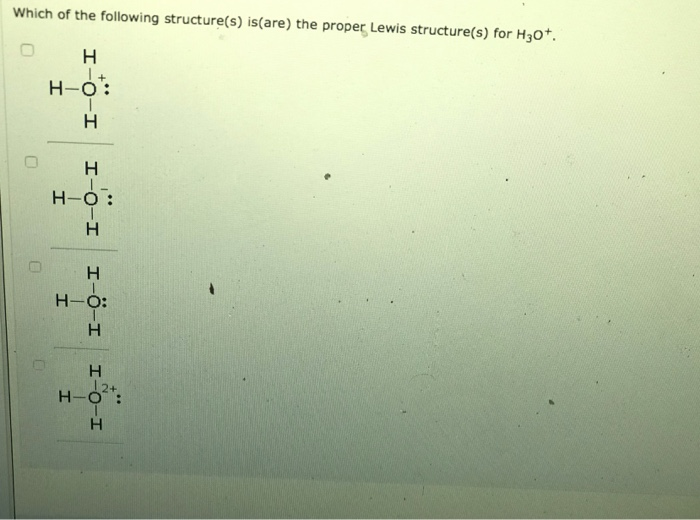
You have to include both bonding pairs and lone pairs. All you need to do is to work out how many electron pairs there are at the bonding level, and then arrange them to produce the minimum amount of repulsion between them. The shape of a molecule or ion is governed by the arrangement of the electron pairs around the central atom. That means that you couldn't use the techniques on this page, because this page only considers single bonds. If you did that, you would find that the carbon is joined to the oxygen by a double bond, and to the two chlorines by single bonds. If you are given a more complicated example, look carefully at the arrangement of the atoms before you start to make sure that there are only single bonds present.įor example, if you had a molecule such as COCl 2, you would need to work out its structure, based on the fact that you know that carbon forms 4 covalent bonds, oxygen 2, and chlorine (normally) 1. The examples on this page are all simple in the sense that they only contain two sorts of atoms joined by single bonds - for example, ammonia only contains a nitrogen atom joined to three hydrogen atoms by single bonds. If you are interested in the shapes of molecules and ions containing double bonds, you will find a link at the bottom of the page. This page explains how to work out the shapes of molecules and ions containing only single bonds. Shapes of molecules and ions containing single bonds


 0 kommentar(er)
0 kommentar(er)
